RESEARCH ARTICLE
New Amino-steroid-anthracenone with Biological Activity Against Ischemia-reperfusion Injury in a Wistar Rat Model
Figueroa-Valverde Lauro1, *, Rosas-Nexticapa Marcela2, *, Mateu-Armand Virginia2, Herrera-Meza Socorro3, Díaz-Cedillo Francisco4, Montano-Tapia Elizabeth2, García-Cervera Elodia1, Pool-Gómez Eduardo1, Hau-Heredia Lenin1, García-Martínez Rolando1, Lopez-Ramos Maria1, Cauich-Carrillo Regina1, Parra-Galindo Perla2
Article Information
Identifiers and Pagination:
Year: 2018Volume: 8
First Page: 10
Last Page: 20
Publisher Id: TOPHARMJ-8-10
DOI: 10.2174/1874143601808010010
Article History:
Received Date: 25/5/2018Revision Received Date: 8/9/2018
Acceptance Date: 15/9/2018
Electronic publication date: 17/10/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective:
The main objective of this study was to evaluate the biological activity of an ASA (Amino-Steroid-Anthracenone derivative) against heart failure caused by the ischemia- reperfusion injury (translated as infarct area).
Methods:
Biological activity exerted by ASA (0.001-100 nM) on infarct area was determined using an ischemia-reperfusion injury model. In addition, to characterize the molecular mechanism involved in the effect exerted by ASA on left ventricular pressure, some drugs such as estrone (0.001-100 nM), tamoxifen (1 nM), butoxamine (1 nM) and ZM-241385 (1 nM) were used.
Results:
The experimental data showed that ASA decreased the infarction area significantly (p = 0.05) compared to estrone. Other results indicated that ASA decreased left ventricular pressure and this effect was inhibited by ZM-241385. In addition, ASA increased cAMP levels in a time-dependent manner compared to control conditions.
Conclusion:
The results showed that ASA decreases ischemia-reperfusion injury (translated as infarct area) via A2 adenosine receptor activation and these phenomena involve changes in cAMP levels.
1. INTRODUCTION
There are some reports which indicate that congestive heart failure has increased in recent years worldwide [1, 2]. It is important to mention that congestive heart failure (translated as myocardial infarction) can induce cardiomyocyte cell death and result in a decrease of cardiac work [3-5]. Some drugs are used for the treatment of this clinical pathology, such as angiotensin-converting-enzyme inhibitors [6], β1-adrenergic agonist or β1-adrenergic blockers [7], diuretics [8], angiotensin-receptor inhibitors [9], calcium sensitizer [10], phosphodiesterase III inhibitors [11], ATP-ase inhibitors [12] and others. However, some of these drugs can produce adverse effects [13-18]; therefore, in the search for a new drug for the treatment of heart failure and ischemia-reperfusion injury, several drugs have been developed such as MK-7145 (potassium channel inhibitor) [19], some heteroaryl-substituted naphthalenes (CYP11B2 inhibitors) [20, 21], BAY-1021189 (guanylate cyclase stimulator) [22], N-benzylcarboxamide (G-protein-coupled receptor kinase 2 inhibitor) [23], a naphthalene-prazosin derivative (calcium channels activation) [24], a guanidine analog (sodium-hydrogen exchanger isoform inhibitor) and others. All these data indicate that several drugs may exert effects on heart failure and ischemia-reperfusion injury; however, the molecular mechanism is very confusing, perhaps, this phenomenon can be due to; 1) differences in the chemical structure of each drug; or 2) to different protocols used in each experiment. Therefore, in this study, the biological activity of an Amino-Steroid-Anthracenone (ASA) (Fig. 1) was evaluated using an ischemia-reperfusion injury model.
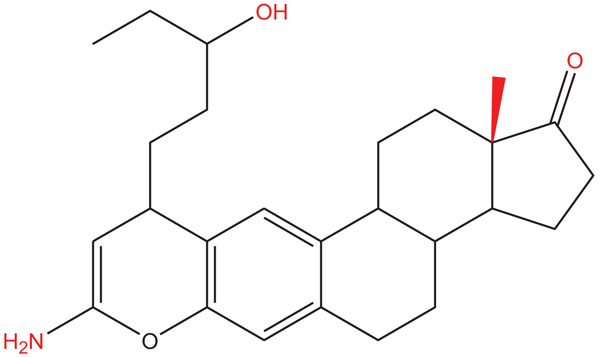 |
Fig. (1). Chemical structure of ASA (8-Amino-10-(3-hydroxy-pentyl)-13a-methyl-3,3a,3b,4,5,10,11b,12,13,13a-decahydro-2H-7-oxa-indeno[5,4-a]anthracen-1-one). |
2. MATERIALS AND METHODS
2.1. General Methods
The compound amino-steroid-anthracenone derivative (ASA; 8-Amino-10-(3-hydroxy-pentyl) -13a-methyl-3, 3a, 3b, 4, 5, 10, 11b, 12, 13, 13a-decahydro-2H-7-oxa-indeno [5,4-a]anthracen-1-one) was prepared using a previously reported method [25].
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal care and use committee of Autonomous University of Campeche (Faculty of Chemical-Biological Sciences) with No. PI-420/12 and were in accordance with the Guide for the Care and Use of Laboratory Animals [26]. Male Wistar rats; weighing 200-250 g were obtained from Pharmacochemistry Laboratory of University Autonomous of Campeche (Faculty of Chemical-Biological Sciences).
2.2. Reagents
It is noteworthy that the drugs involved in this study were dissolved in methanol and from this solution, all dilutions were done using a Krebs-Henseleit* solution (v/v).
2.3. Biological Activity
2.3.1. Experimental Design I
Animals were anesthetized with pentobarbital (50 mg/Kg body weight) via intraperitoneal administration. After the animal was opened by a thoracic abdominal laparotomy, the heart was perfused via retrograde with the Krebs-Henseleit solution through a non-circulating perfusion system with a constant flow rate. It is important that the study population involved in each group was n = 9. *Krebs-Henseleit system was composed of the following substances in mmol concentrations per liter; 117.8 NaCl; 6 KCl; 1.75 CaCl2; 1.2 NaH2PO4; 1.2 MgSO4; 24.2 NaHCO3; 5 glucose, and 5 sodium pyruvate [27]. The solution was then bubbled with a mixture of O2/CO2 (95:5/5%) and the mixture was regulated to a pH of 7.4 (37°C). The coronary flow (10 mL/min) was adjusted with a peristaltic pump for an equilibration period of 15 min.
2.3.2. Ischemia-reperfusion Model
It is important to mention that after the equilibrium time, the hearts underwent a period of ischemia for a duration of 40 mins due to the closure of the perfusion system as indicated in some reports [24], in the absence (control) or in the presence of each of the drugs involved in this study. Following this, the system was restarted, and the hearts were reperfused for another 40 mins with Krebs-Henseleit solution.
2.4. Biological Evaluation
2.4.1. Step I
2.4.1.1. Effects Induced by the Compound
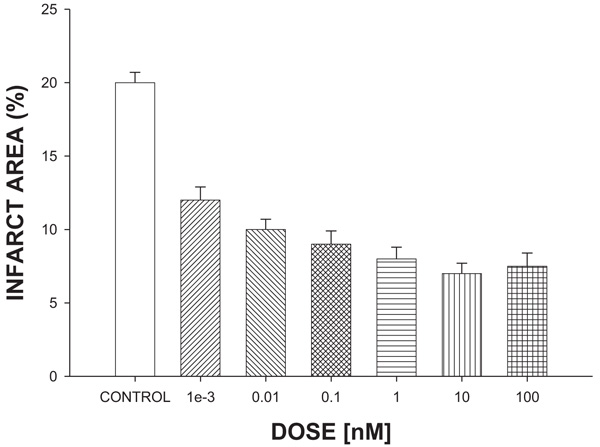
Amino-Steroid-Anthracenone derivative (ASA) against infarct area. The effect induced by ASA on ischemia-reperfusion injury (translated as infarction area*) was evaluated at a dose range of 0.001-100 nM.
2.4.1.2. Biological Activity Produced by Estrone and ASA Against Infarct Area
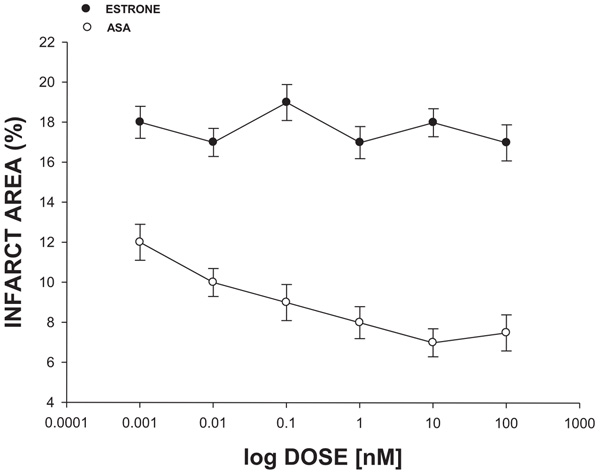
The effect induced by estrone (has a steroid nucleus similar to ASA) at a dose range of 0.001-100 nM on the ischemia-reperfusion injury (translated as infarction area*) was determined.
*The infarction area was determined through a previously reported planimetry method [28] using an image-Pro Plus 4.5 software (Media cybernetics, Silver Spring, USA).
2.4.2. Step II
2.4.2.1. Effects Induced by ASA on Left Ventricular Pressure Through Estrogen Receptor Activation
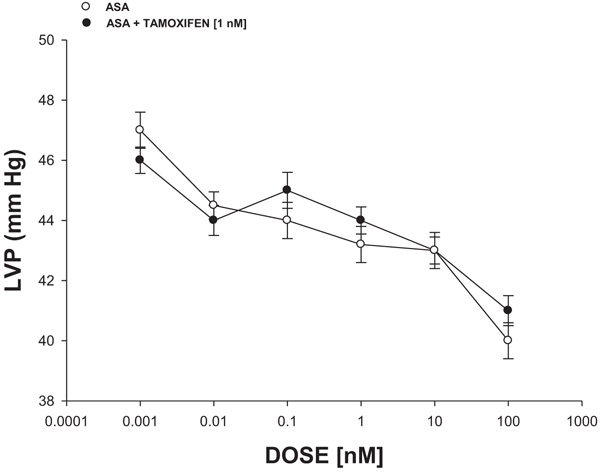
Intracoronary boluses (50 μL) of the ASA (0.001 to 100 nM) were administered and the corresponding effect on the left ventricular pressure was determined. The dose-response curve (control) was repeated in the presence of tamoxifen (estrogen inhibitor) at a concentration of 1 nM (duration of pre-incubation with tamoxifen was 10 min equilibration period).
2.4.2.2. Effects Induced by the ASA on Left Ventricular Pressure Through B2-Adrenergic Receptor Stimulation
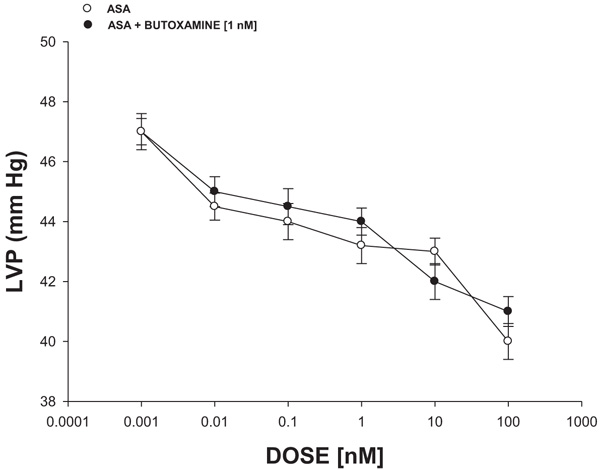
Intracoronary boluses (50 μL) of ASA (0.001 to 100 nM) were administered and the corresponding effect on the left ventricular pressure was determined. The dose-response curve (control) was repeated in the presence of butoxamine (β2-selective beta blocker) at a concentration of 1 nM (duration of pre-incubation with butoxamine was 10 min equilibration period).
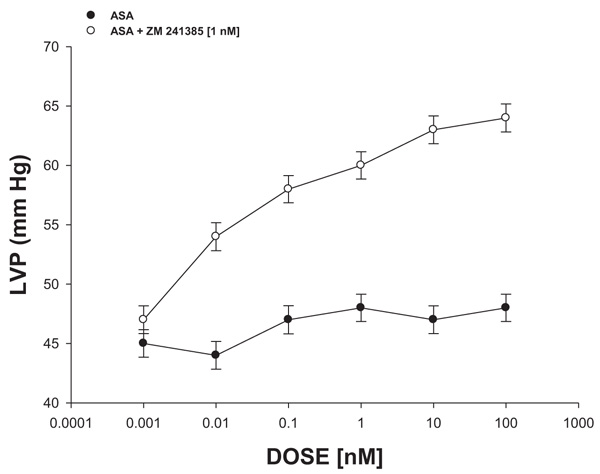
2.4.2.3. Effect Exerted by ASA on Left Ventricular Pressure Through A2-Purinergic Receptor Activation
The boluses (50 μL) of ASA (0.001 to 100 nM) were administered and the corresponding effect on the left ventricular pressure was evaluated. The bolus injection administered was done at the point of cannulation. The dose-response curve (control) was repeated in the presence of ZM-241,385 (adenosine A2 antagonist) at a concentration of 1 nM (duration of the pre-incubation with ZM-241,385 was for a period of 10 min).
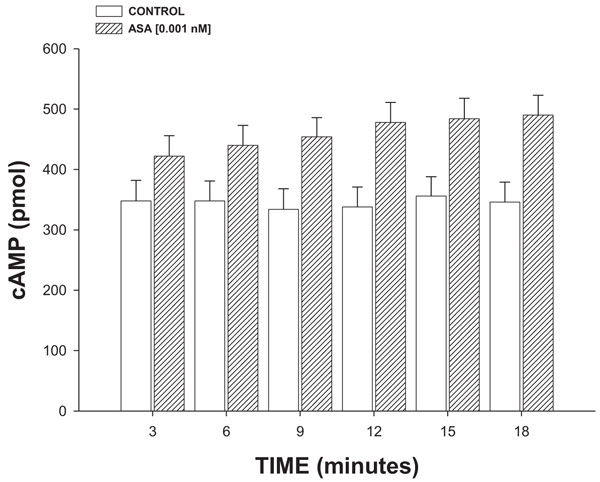
2.4.2.4. Effect Exerted of ASA on cAMP Concentration
Isoproterenol (β2 adrenoreceptor agonist) was perfused at a dose of 100 nM/min on the heart for 3, 6, 9, 12 or 18 minutes and its effect on cAMP levels was determined using a previously reported method [24]. The results obtained were compared with the biological activity exerted by ASA (1 nM/min) on the concentration of cAMP as against the controls.
2.5. Statistical Analysis
The results were expressed as average ± SE, using each heart (n = 9) as its own control. In addition, the results were analyzed via Analysis of Variance using the SPSS 12.0 program The differences in the values found were determinates of p ≤ 0.05 [29].
3. RESULTS
In this study, the biological activity induced by an Amino-Steroid-Anthracenone derivative (ASA) with biological activity against ischemia-reperfusion injury was evaluated.
3.1. First Stage
3.1.1. Effect Exerted of ASA on Ischemia/Reperfusion Injury
The results (Fig. 2) showed that the ASA reduces infarct size significantly (p = 0.05) expressed as a percentage of the area at a risk compared with vehicle-treated hearts (control).
3.1.2. Biological Activity Induced by Both Estrone and ASA on Ischemia/Reperfusion Injury
The experimental data (Fig. 3) showed that the infarct area was significantly reduced (p = 0.05) in the presence of ASA compared with estrone in a dose-dependent manner (0.001 to 100 nM).
3.2. Second Stage
3.2.1. Effects Induced by ASA on Left Ventricular Pressure Through Estrogen Receptor Activation
Fig. 4 shows that ASA (0.001 to 100 nM) decreased the left ventricular pressure in a dose-dependent manner, both in the absence or the presence of tamoxifen (1 nM).
3.2.2. Effects Induced by the ASA on Left Ventricular Pressure Through Β2-Adrenergic Receptor Stimulation
The experimental data (Fig. 5) indicated that PDC (0.001 to 100 nM) decreased the left ventricular pressure in a dose-dependent manner in the absence or the presence of butoxamine [1 nM].
3.2.3. Effect Exerted by ASA on Left Ventricular Pressure Through A2-Purinergic Receptor Activation
The results shown in Fig. 6 showed that ASA (0.001 to 100 nM) decreased the left ventricular pressure in a dose-dependent manner and this effect was inhibited in the presence of ZM-241,385 [1 nM].
3.2.4. Effect Exerted of ASA on cAMP Concentration
The results (Fig. 7) showed that ASA (1 nM/min) increased the cAMP levels in a time-dependent manner (3-18 min).
4. DISCUSSION
There are several drugs that have been used to treat heart failure resulting from myocardial ischemia [30, 31]. Nevertheless, there is scarce information about the effects of the Amino-Steroid-Anthracenone (ASA) on this clinic pathology. Therefore, in this study, the biological activity of an ASA against myocardial ischemia translated as infarct area was evaluated using an ischemia-reperfusion injury model [32]. The results showed that ASA reduces infarct size (expressed as a percentage of the area at risk) to different doses compared with conditions control. In order to evaluate whether the biological activity exerted by ASA could be related to the steroid nucleus involved in their chemical structure, in this study, estrone was used as a pharmacological tool. The experimental data showed that ASA reduced infarct size, in a dose-dependent manner, compared with estrone; these data suggest that possibly the pyran ring or the functional groups bound to pyran ring of ASA could be responsible for its biological activity. This finding is supported by some reports which indicate that several pyran derivatives can exert an effect on the cardiovascular system [33-35] and can reduce the area infarction by inducing changes in left ventricular pressure [36, 37]. Analyzing these data, in this study, the effect exerted by ASA on left ventricular pressure was evaluated; the results showed that ASA decreases the left ventricular pressure in a dose-dependent manner compared with estrone and conditions control.
On the other hand, in the search for a molecular mechanism involved in biological activity induced by ASA on left ventricular pressure, earlier reports indicated that several compounds can induce changes in the left ventricular pressure through estrogen receptor activation [38, 39]. To evaluate this hypothesis, in this study, the biological activity exerted by ASA on left ventricular pressure in the absence or presence of tamoxifen was evaluated. The results showed that ASA decreases left ventricular pressure and this effect was not inhibited by tamoxifen; this phenomenon indicated that molecular mechanism involved in the biological activity of ASA on left ventricular pressure was not via estrogen-receptor activation.
Since earlier reports indicated that some compounds can exert changes on left ventricular pressure through β2-adrenergic receptor activation [40], in this study, the biological activity of ASA on left ventricular pressure was evaluated in the absence or presence of butoxamine (β2-adrenergic receptor inhibitor) [41]. The results showed that ASA decreased left ventricular pressure and this effect was not inhibited by butoxamine; this phenomenon indicated that molecular mechanism involved in the biological activity of ASA on left ventricular pressure was not via β2-adrenergic receptor activation.
On the other hand, other reports indicated that some substances can exert changes in the pressure of the left ventricle through A2-adenosine receptor and this phenomenon may result in a decrease in the infarct area [41, 42]. Therefore, in this study, the effect exerted by ASA on left ventricular pressure was determined, both in the absence or presence of ZM241385 (A2-receptor adenosine antagonist) [43] The results showed that ASA decreases left ventricular pressure and this effect was inhibited by ZM241385. These data indicated that the effect exerted by ASA on left ventricular pressure was via A2-adenosine receptor activation.
On the other hand, it is important to mention that the interaction of some substances with A2-adenosine receptor could involve the synthesis and release of cAMP [44]. Therefore, in this study, an experimental alternative was carried out to evaluate the possibility that ASA could exert changes on cAMP levels; the results showed that ASA significantly increases the cAMP concentration compared with the conditions control.
CONCLUSION
The Amino-Steroid-Anthracenone derivative (ASA) is a particularly interesting compound because the biological activity exerted against ischemia-reperfusion injury involves a molecular mechanism different in comparison with other drugs; this phenomenon may constitute a novel therapy for ischemia-reperfusion injury translated as a decrease in the heart failure; however, it is important to carry out toxicity studies of this compound to rule out any adverse effect.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal care and use committee of Autonomous University of Campeche (Faculty of Chemical-Biological Sciences) with No. PI-420/12 and were in accordance with the Guide for the Care and Use of Laboratory Animals.
HUMAN AND ANIMAL RIGHTS
No humans were used in the experiments. All the reported experiments involving animals used in the study were in accordance with the Animal Ethics Committee of Autonomous University of Campeche (Faculty of Chemical-Biological Sciences) with No. PI-420/12.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.