RESEARCH ARTICLE
Hepatoprotective Activity of Tectona grandis Against CCl4-Induced Hepatic Damage in Rats
Geeta Deswal1, *, Kumar Guarve1, Priyanka Kriplani1, Ashwani K. Dhingra1, Bhawna Chopra1, Jaspreet Sidana2
Article Information
Identifiers and Pagination:
Year: 2019Volume: 9
First Page: 5
Last Page: 11
Publisher Id: TOPHARMJ-9-5
DOI: 10.2174/1874143601909010005
Article History:
Received Date: 30/5/2019Revision Received Date: 24/9/2019
Acceptance Date: 10/10/2019
Electronic publication date: 15/11/2019
Collection year: 2019
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Literature reports numerous causes for liver damage, which mainly include viral hepatitis (most commonly hepatitis B), cirrhosis, cell stress, hepatic damage by NSAIDs or alcohol. In the present study, methanolic extracts of Tectona grandis leaves were evaluated for hepatoprotective activity against CCl4 induced liver damage in rats.
Methods:
Hepatic injury in rats was carried out using the CCl4-induced hepatotoxic model. Methanolic extracts of Tectona grandis were administered orally at two different doses (200mg/kg & 400mg/kg) daily. The biochemical parameters (SGOT, SGPT, ALP, and serum bilirubin) were estimated using Reitman and Frankel's method in addition Kind King’s method.
Results:
The preliminary phytochemical studies confirmed the existence of saponins, carbohydrates, tannins, and flavonoids. CCl4 treated group boost the concentrations of Serum Glutamate Pyruvate Transaminase (SGPT), Serum Glutamate Oxaloacetate Transaminase (SGOT), Alkaline Phosphate (ALP) and serum bilirubin as compared to control group (rats treated with vehicle). The methanolic extract of plant (200 mg/kg & 400 mg/kg) and standard drug silymarin (100 mg/kg) produced a significant decrease in raised levels of these enzymes as compared to control.
Conclusion:
The results clearly indicate that Tectona grandis leaves have notable hepatoprotective activity in rats hepatic damage induced by CCl4.
1. INTRODUCTION
The liver is a large, solid, gland situated in the right upper quadrant of the abdominal cavity. It is the chief organ of the body that metabolizes carbohydrate, lipid, protein, processing of drugs and hormones. It has the great capacity to detoxify glycogen, vitamin A, D & B12 storage, coagulation factor production, growth factor and hormones, biomarkers essential for digestion (bile) [1]. Now a days, liver damage is of great concern. Many reasons were reported in the literature for liver damage i.e. hepatitis B (viral hepatitis), cirrhosis and hepatic damage caused by drugs like NSAIDs (acetaminophen) or excess consumption of alcohol. It is well studied that initial injury of the liver may be associated with cell stress, specific immune reaction or direct mitochondrial inhibition which leads to Mitochondrial Permeability Transition (MPT).
MPT is caused by direct cell stress through intrinsic pathway [2]. Activation of pro-apoptotic proteins and intracellular stress on cascades is involved in the intrinsic pathway. The death receptor-mediated extrinsic pathway alternatively initiates the MPT which is activated by fast binding to the death receptor. Hepatotoxicity may occur idiosyncratically due to various amplification mechanisms. Production of reactive oxygen species is considered as a crucial factor leading to oxidative damage of tissues by releasing free radicals [3]
CCl4 is one of the potent hepatotoxins which cause centrilobular hepatic necrosis which is mainly used for liver fibrosis animal models. It is marked that CCl4 is activated by CYP450 to trichloromethyl free radical and provokes Kupffer cells to produce ROS e.g. H2O2, OH, and O2- that cause liver damage [4-6]. Ayurveda includes many medicinal plants which have well known hepatoprotective potential [7]. One of the comprehensive examples is silymarin, which is isolated from Silybum marianum [8, 9] and well known for its hepatoprotective activity.
The methanol extracts of Tectona grandis leaves were investigated against CCl4 induced hepatic damage in adult Wistar rats. Tectona grandis (Lamiaceae) is universally named as sangwan in Hindi, teek in France, jati in Indonesia, java teak in Germany, may ask in Laos and Thailand. It is native to the southeast of south Asia, Indonesia, mostly India, Myanmar, and Malaysia, but it is cultivated and naturalized in various countries i.e. Africa and the Caribbean. Myanmar is the main hub of Tectona grandis which accounts for about one-third of the total world’s production. The leaves of Tectona grandis mainly used for depurative, hemostatic, anti-inflammatory, leprosy, vulnerary, skin diseases, stomatitis, pruritis, indolent ulcers, hemoptysis, hemorrhages, vitiated conditions of pitta [10-14].
2. MATERIALS AND METHODS
2.1. Plant Material
The leaves of the plant Tectona grandis were collected from the Herbal Nature Park, Chuharpur, Forest Division, Yamunanagar, Haryana. Taxonomically, the plant was authenticated by Dr. H. B. Singh, Scientist Incharge and Head, Raw materials Herbarium and Museum, at National Institute of Science Communication and Information Resources (NISCAIR), New Delhi with a voucher specimen number NISCAIR/ RHMD/Consult/-2010-11/1619/217. After collection, the leaves were washed thrice with water to remove dust or debris and were air-dried. After drying completely, the leaves were ground to a coarse powder using a blender, then passed through the sieve of 40 mesh size and stored in a well-closed container.
2.2. Plant Extract Preparation
Air-dried leaf powder of Tectona grandis (500g) was subjected to Soxhlet extraction by using methanol as solvent. The extract was further concentrated by using Buchi rotary vacuum evaporator (Gupta scientific store, Ambala) and then evaporating to dryness on the water-bath.
2.3. Phytochemical Screening
Phytochemical screening of extract was done to detect the presence of various chemical constituents like carbohydrates, amino acids, glycosides, alkaloids, lipids, fats, fixed oils, saponins, and flavonoids [15].
2.4. Experimental Animals
Adult Wistar rats of either sex (100-150g) were selected for the experimental study. The procurement of rats was done from the animal house of M.M. College of Pharmacy Mullana, (Ambala), Haryana. The animals were kept and maintained under laboratory control conditions of humidity (60 ± 1%), temperature (21.5°C ± 22°C) and 12 hour light or dark cycle. Free access to water and food was allowed to rats. Experiment protocols and procedures employed in the study were approved by the Institutional Animal Ethics Committee of M. M. University Mullana (Ambala), India (Reg. No. 1355/AC/10/ CPCSEA) and confirmed with the guideline of Committee for the purpose of Control and Supervision on experiments on animals.
2.5. Toxicity Studies
The acute toxicity studies of methanolic extract of leaves of Tectona grandis were conducted by using adult Wistar rats of either sex (100-150g) maintained under approved husbandry conditions. In accordance with the up and down method of CPCSEA for toxicity studies, the animals fasted for 12 hours before the start of the experiment. Tectona grandis extract was administered orally at different doses (50-1000mg/kg). The experimental animals were kept under observation for behavioral changes from the first hour of drug intake up to seven days. The parameters, for example, hyperactivity, convulsions, hypothermia, sedation, mortality, and grooming were recorded for the doses of 200 mg/kg/day and 400 mg/kg/day [16].
2.6. Experimental Design
Adult Wistar rats of either sex (100-150gm) were divided into five groups containing six animals in each group. Group I animals were considered as control who received only the vehicle for ten days at a dose of 1ml/kg (p.o.). Group II animals were considered as positive control and received a dose of carbon tetrachloride dispersed in sterile olive oil (1:1 v/v, 2ml/kg, i.p.) for ten days every 72 hours. Standard drug silymarin (100 mg/kg) was given to group III along with carbon tetrachloride dispersed in sterile olive oil (1:1 v/v, 2ml/kg, i.p.) for 10 days. Group IV-V received methanolic extract at the dose 200 and 400 mg/kg/day (dispersed in 0.5% sodium carboxymethyl cellulose) and carbon tetrachloride dispersed in sterile olive oil (1:1 v/v, 2 ml/kg) for 10 days. Overnight rats were fasted and sacrificed by using ethyl anesthesia at the end of the experiment. The blood samples were collected separately into the sterilized dry centrifuge tubes and separated serum was analyzed for determination of the liver enzymes markers. For histopathological studies, the liver is removed and kept in 10% formalin solution [17].
2.7. Estimation of Biochemical Markers
Reitman and Frankel's method is used for the estimation of SGOT and SGPT. Kind King’s method is used for the estimation of SALP and serum bilirubin. Jendrassik and Grof’s method and cholesterol oxidase/peroxidases method are used for the determination of total bilirubin and total protein, respectively [18-21].
2.8. Histopathological Studies
10% neutral formalin solution was used to fix the liver from rats, further dehydrated with alcohol and embedded in paraffin. For light microscope analysis, fine sections mounted on glass slides were counterstained with hematoxylin and eosin.
2.9. Statistical Analysis
Biochemical estimation results were marked for analysis of significant intergroup difference. Each parameter was analysed separately and one-way analysis of variance (ANOVA) was carried out. Dunnet's test was used for individual comparisons. Statistically significant results at P<0.05 were considered [22].
3. RESULTS
3.1. Phytochemical Screening
Phytochemical screening shows the presence of steroids, phenolic components, tannins, flavanoids and saponin in methanolic extracts of Tectona grandis leaves. The results are depicted in (Table 1).
3.2. Toxicity Studies
Oral administration of the methanolic extract of Tectona grandis at a dose of 1000 mg/kg did not indicate any adverse effects or mortality when observed for 04 hrs and every-day for the next ten days. Data obtained from this and pilot study two doses i.e. 200 and 400 mg/kg were selected for further study.
3.3. Effect on Biochemical Markers
CCl4 treated Group II animals showed a steep boost in the levels of enzymes i.e. SGOT, SALP, SGPT, and serum bilirubin as compared to group I (untreated). Silymarin and Tectona grandis extract (200mg/kg and 400mg/kg) treated group (III-IV) showed a sharp fall in the levels of enzymes as compared to group II i.e. CCl4 treated group (Table 2 & Fig. 1).
3.4. Effect on Liver Histopathology
The microscopic histopathological evaluation of livers from the control group, CCl4 treated group, standard drug silymarin, and methanolic extract have revealed the following observations.
3.5. Normal Control Group
The typical lobular arrangement is observed in rat liver. Hepatocytes are arranged in lobules as cords radiating around the terminal that is centrally placed hepatic veins. Hepatocytes seen are of uniform size having polyherbal shape and large nuclei centrally located. The cytoplasm is with a fine basophilic granularity is eosinophilic. Portal tracts comprising terminal branches hepatic portal vein and hepatic artery in fibrous stoma at the periphery are seen (Fig. 2).
3.6. CCl4 Treated Group
The rat liver tissue with the disturbed lobular arrangement is observed. Early necrotic and degenerative alterations extending lobules are visible. There are steatotic alterations and ballooning degenerations in hepatocytes. Some amount of fibrosis seen in portal tracts. Toxic injury with fibrotic alterations and mild necrosis is observed in the liver (Fig. 3).
3.7. Standard Silymarin Group
The rat liver showed liver tissue with the typical lobular arrangement. Variable size hepatocytes were observed and there is an increase in fibrous connective tissues. Mild hepatotoxicity is indicated in the liver (Fig. 4).
3.8. Methanolic Extract Treated Group
Rat liver showed liver tissue with the typical lobular arrangement. Few hepatocytes show steatotic accumulation. The liver with minimal sign of hepatotoxicity is observed. (Fig. 5 and Fig. 6).
| Sr. No. | Components | Methanol extract |
|---|---|---|
| 1 | Carbohydrates | - |
| 2 | Proteins and amino acids | - |
| 3 | Fixed oil and Fats | - |
| 4 | Alkaloids | - |
| 5 | Glycosides | - |
| 6 | Steroids | + |
| 7 | Phenolic compounds and Tannins | + |
| 8 | Flavonoids | + |
| 9 | Saponins | + |
| Treatment | Parameters | |||
|---|---|---|---|---|
| SGPT | SGOT | SALP | Serum Bilirubin | |
| Normal control | 35.83±1.74 | 44.17±0.79 | 26.08±0.47 | 2.18±0.051 |
| Positive control | 132.8±2.25 | 152.2±1.95 | 90.35±3.19 | 12.5±0.63 |
| Standard+CCl4 | 42.83±2.71 | 59.33±3.30 | 44.13±0.91 | 5.23±0.55 |
| Tectona grandis (200mg/kg) +CCl4 | 48.67±2.36 | 62.17±2.77 | 47.68±3.21 | 5.85±0.43 |
| Tectona grandis (400mg/kg) +CCl4 | 59.83±1.70 | 67.66±3.38 | 60.74±1.02 | 6.28±0.28 |
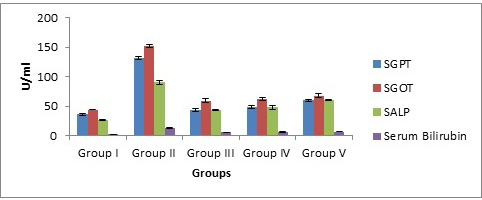 |
Fig. (1). Effect of methanolic extract of Tectona grandis on biochemical parameters in experimental animal. |
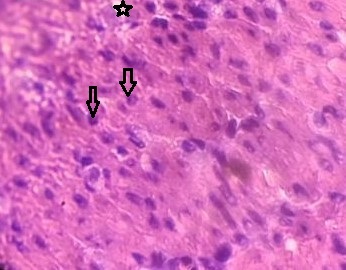 |
Fig. (2). Portal tracts comprising of terminal branches hepatic portal vein and hepatic artery in fibrous stoma at the periphery. |
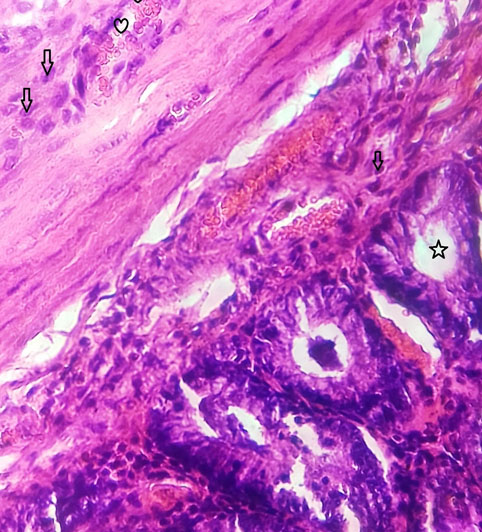 |
Fig. (3). Toxic injury with fibrotic alterations and mild necrosis is observed in the liver. |
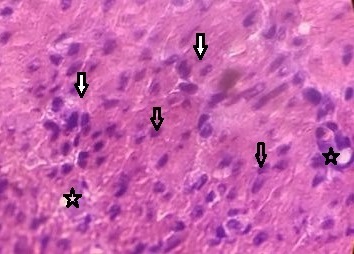 |
Fig. (4). Mild hepatotoxicity is indicated in the liver. |
 |
Fig. (5). Liver with minimal sign of hepatotoxicity. |
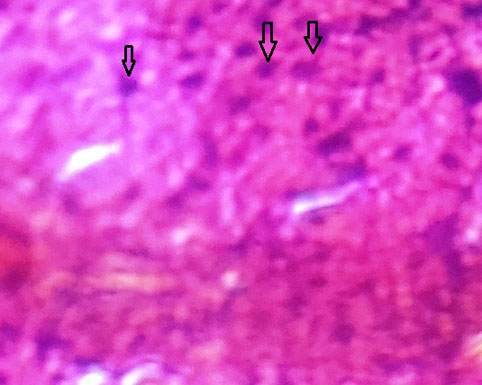 |
Fig. (6). Liver with minimal sign of hepatotoxicity. |
4. DISCUSSION
CCl4 is commonly used hepatotoxin for the experimental study of liver disorder. CCl4 induced liver cell injury involves CCl4 biotransformation caused by cytochrome P450 leading to the production of trichloromethyl free radical which causes peroxidative degradation in adipose tissue causing hepatocyte fatty infiltration. Lipid peroxidation is caused by trichlo- romethyl free radicals in the presence of oxygen produced by metabolic leakage from mitochondria. All these changes inhibit the damage of hepatic tissue and loss of integrity of cell membrane. CCl4 increases SGPT, SGOT and bilirubin level, and induced hepatotoxicity.
In the present study, Tectona grandis was selected for hepatoprotective activity owing to its traditional use. The liver function is assessed by determining the activities of SGOT, SGPT, SALP, and serum bilirubin i.e. enzymes present in the cytoplasm. In the case of hepatotoxicity, the enzymes move into the bloodstream and their amount confirms the liver damage extent.
Levels of bilirubin indicate the severity of necrosis and its increased levels indicate conjugation; binding and excretory power of hepatocyte, whereas the effectiveness of extracts is indicated by decreased levels of bilirubin in liver damage initiated by chemicals.
Phytochemical tests of methanolic extract of leaves of Tectona grandis indicated the existence of saponins, flavo- noids, and phenolic compounds. Administration of methanolic extract showed a substantial decrease in SGPT, SGOT, SALP and serum bilirubin level (p <0.05) at a dose of 400mg/kg in comparison with 200mg/kg. Similarly, treatment with hepatoprotective drug such as silymarin showed a significant decrease in SGPT, SGOT, SALP, and serum bilirubin levels. Therefore, it was reported that Tectona grandis extract treatments normalize few morphological features of the liver in rats with CCl4 induced hepatitis.
CONCLUSION
Form the above preliminary study, we can conclusively state that the methanolic extract of Tectona grandis leaves has shown remarkable hepatoprotective activity. The significant hepatoprotective effect of the methanolic extracts may be due to the presence of flavonoids, rutin and quercetin or their synergistic properties. However, further study & biochemical investigations are required to isolate & identify the active hepatoprotective constituents present in the plants as well as elucidating their mechanism of action.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Experiment protocols and procedures used in this study were approved by the Institutional Animal Ethics Committee of M. M. University Mullana (Ambala), India, [Reg. No. 1355/AC/10/CPCSEA].
HUMAN AND ANIMAL RIGHTS
No humans were used for the studies. The study conformed to the guideline of Committee for the purpose of Control and Supervision on Experiments on Animals.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the M. Pharmacy thesis available at M. M. University Mullana Ambala with registration no. 09-BPM-146.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.




